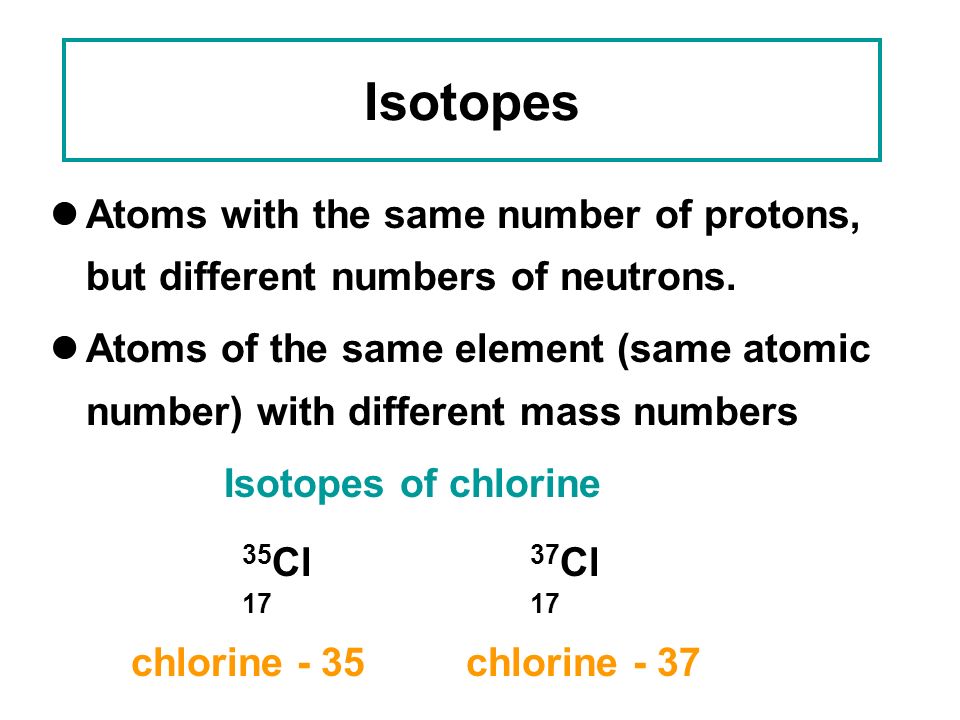
Atoms with the same number of protons but different numbers of neutrons are called isotopes. Some elements only have one naturally occurring isotope, while others such as carbon have two or more. In a naturally occurring sample, isotopes of each element are present in a certain percentage amount called the percent natural abundance. Atoms are made of protons, neutrons, and electrons. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. A neutral atom has the same number of protons and electrons (charges cancel each other out). An ion has an unequal number of protons and electrons.
Isotopes
Concept Question: What is an isotope?
The basic structure of the atom is a nucleus surrounded by electro-magnetic fields in which moving electrons reside. Inside the nucleus reside nucleons: neutrons and protons. When an atom is characterized by a unique number of nucleons, we refer to it as a nuclide. Different numbers of neutrons and/or protons result in different nuclides.
If two atoms have different numbers of protons, they are different elements. However, if two atoms have the same number of protons, but different numbers of neutrons we refer to them as isotopes.
Two terms we use to identify nuclides (isotopes) are atomic number and mass number. Two atoms with the same atomic number, but different mass numbers (same number of protons, different number of neutrons), are called isotopes, or isotopic nuclides.
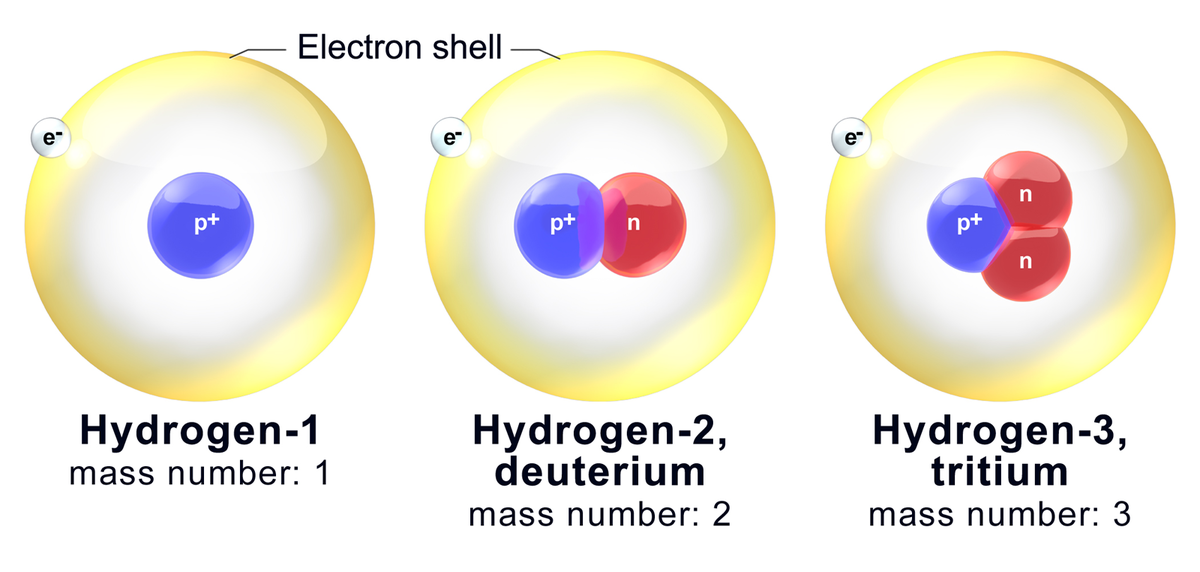
Having different numbers of neutrons changes the mass of these atoms, so isotopes have slight variations in their physical and chemical behavior. Some elements have many different isotopes, some only have a few, and some have no stable isotopes at all.
A particular isotope can be described in several ways. If we were discussing the isotopes of carbon and wanted to specify the isotope with a mass number (A) of 12 we would say 'carbon twelve,' and this could be written as carbon-12, or in a symbolic form with the mass number as a superscript: 12C. This symbolic form can also include the atomic number (Z) as a subscript, as in .
Learning Objectives
- Define atomic number.
- Define mass number.
- Determine the number of protons, neutrons, and electrons in an atom.
It's important to be able to distinguish atoms of one element from atoms of another element. Elements are pure substances that make up all other matter, so each one is given a unique name. The names of elements are also represented by unique one- or two-letter symbols, such as (ce{H}) for hydrogen, (ce{C}) for carbon, or (ce{He}) for helium. However, it would more powerful if these names could be used to identify the numbers of protons and neutrons in the atoms. That's where atomic number and mass number are useful.
Atomic Number
Scientists distinguish between different elements by counting the number of protons in the nucleus (Table (PageIndex{1})). If an atom has only one proton, we know that it's a hydrogen atom. An atom with two protons is always a helium atom. If scientists count four protons in an atom, they know it's a beryllium atom. An atom with three protons is a lithium atom, an atom with five protons is a boron atom, an atom with six protons is a carbon atom . . . the list goes on.
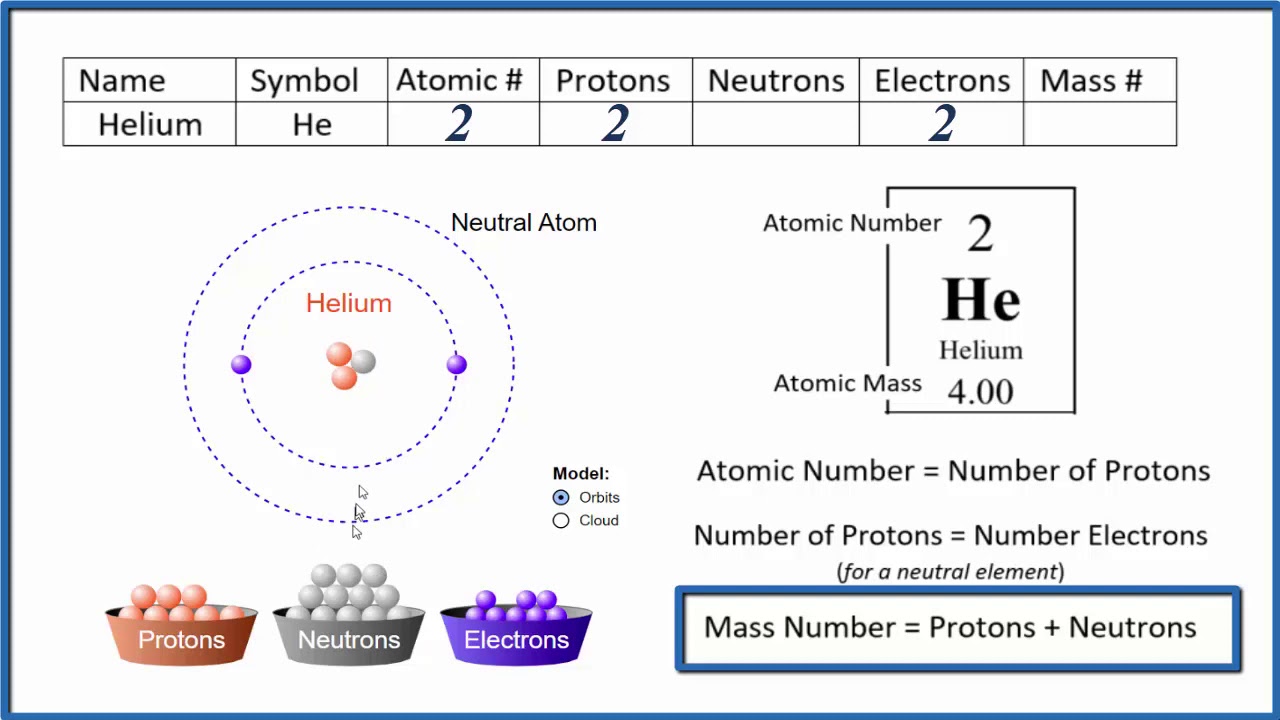
Since an atom of one element can be distinguished from an atom of another element by the number of protons in its nucleus, scientists are always interested in this number, and how this number differs between different elements. The number of protons in an atom is called its atomic number ((Z)). This number is very important because it is unique for atoms of a given element. All atoms of an element have the same number of protons, and every element has a different number of protons in its atoms. For example, all helium atoms have two protons, and no other elements have atoms with two protons.
| Name | Protons | Neutrons | Electrons | Atomic Number (Z) | Mass Number(A) |
|---|---|---|---|---|---|
| Hydrogen | 1 | 0 | 1 | 1 | 1 |
| Helium | 2 | 2 | 2 | 2 | 4 |
| Lithium | 3 | 4 | 3 | 3 | 7 |
| Beryllium | 4 | 5 | 4 | 4 | 9 |
| Boron | 5 | 6 | 5 | 5 | 11 |
| Carbon | 6 | 6 | 6 | 6 | 12 |
Of course, since neutral atoms have to have one electron for every proton, an element's atomic number also tells you how many electrons are in a neutral atom of that element. For example, hydrogen has an atomic number of 1. This means that an atom of hydrogen has one proton, and, if it's neutral, one electron as well. Gold, on the other hand, has an atomic number of 79, which means that an atom of gold has 79 protons, and, if it's neutral, 79 electrons as well.
How To Find Protons Neutrons And Electrons
Neutral Atoms
Atoms are neutral in electrical charge because they have the same number of negative electrons as positive protons (Table (PageIndex{1})). Therefore, the atomic number of an atom also tells you how many electrons the atom has. This, in turn, determines many of the atom's chemical properties.
Mass Number
The mass number ((A)) of an atom is the total number of protons and neutrons in its nucleus. The mass of the atom is a unit called the atomic mass unit (left( text{amu} right)). One atomic mass unit is the mass of a proton, or about (1.67 times 10^{-27}) kilograms, which is an extremely small mass. A neutron has just a tiny bit more mass than a proton, but its mass is often assumed to be one atomic mass unit as well. Because electrons have virtually no mass, just about all the mass of an atom is in its protons and neutrons. Therefore, the total number of protons and neutrons in an atom determines its mass in atomic mass units (Table (PageIndex{1})).

Consider helium again. Most helium atoms have two neutrons in addition to two protons. Therefore the mass of most helium atoms is 4 atomic mass units ((2 : text{amu}) for the protons + (2 : text{amu}) for the neutrons). However, some helium atoms have more or less than two neutrons. Atoms with the same number of protons but different numbers of neutrons are called isotopes. Because the number of neutrons can vary for a given element, the mass numbers of different atoms of an element may also vary. For example, some helium atoms have three neutrons instead of two (these are called isotopes and are discussed in detail later on).
Why do you think that the 'mass number' includes protons and neutrons, but not electrons? You know that most of the mass of an atom is concentrated in its nucleus. The mass of an atom depends on the number of protons and neutrons. You have already learned that the mass of an electron is very, very small compared to the mass of either a proton or a neutron (like the mass of a penny compared to the mass of a bowling ball). Counting the number of protons and neutrons tells scientists about the total mass of an atom.
[text{mass number} : A = left( text{number of protons} right) + left( text{number of neutrons} right)]
An atom's mass number is very easy to calculate, provided that you know the number of protons and neutrons in an atom.
Example 4.5.1
Atoms With The Same Number Of Protons And A Different Number Of Neutrons Are
What is the mass number of an atom of helium that contains 2 neutrons?
Solution
(left( text{number of protons} right) = 2) (Remember that an atom of helium always has 2 protons.)
(left( text{number of neutrons} right) = 2)
(text{mass number} = left( text{number of protons} right) + left( text{number of neutrons} right))
(text{mass number} = 2 + 2 = 4)
A chemical symbol is a one- or two-letter designation of an element. Some examples of chemical symbols are (ce{O}) for oxygen, (ce{Zn}) for zinc, and (ce{Fe}) for iron. The first letter of a symbol is always capitalized. If the symbol contains two letters, the second letter is lower case. The majority of elements have symbols that are based on their English names. Quicktime player download for mac 2019. However, some of the elements that have been known since ancient times have maintained symbols that are based on their Latin names, as shown in Table (PageIndex{2}).
| Chemical Symbol | Name | Latin Name |
|---|---|---|
| (ce{Na}) | Sodium | Natrium |
| (ce{K}) | Potassium | Kalium |
| (ce{Fe}) | Iron | Ferrum |
| (ce{Cu}) | Copper | Cuprum |
| (ce{Ag}) | Silver | Argentum |
| (ce{Sn}) | Tin | Stannum |
| (ce{Sb}) | Antimony | Stibium |
| (ce{Au}) | Gold | Aurum |
| (ce{Pb}) | Lead | Plumbum |
Summary
Atoms With The Same Number Of Protons Are
- Elements are pure substances that make up all matter, so each one is given a unique name.
- The names of elements are also represented by unique one- or two-letter symbols.
- Each element has a unique number of protons. An element's atomic number is equal to the number of protons in the nuclei of any of its atoms.
- The mass number of an atom is the sum of the protons and neutrons in the atom.
- Isotopes are atoms of the same element (same number of protons) that have different numbers of neutrons in their atomic nuclei.
Contributions & Attributions

This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
Marisa Alviar-Agnew (Sacramento City College)
Vmware tools for mac os high sierra download. Henry Agnew (UC Davis)
