Atomic Number Of Compact Bone
Like many of the transition elements, copper has a colored ion. Copper typically forms a bluish green solution. Copper (Cu) has two valences Cu I (cuprous) has one valence electron and Cu II (cupric) has two valence electrons. Copper was one of the earliest known metals, having reportedly been mined for. Copper offers a relatively large number of radioisotopes that are potentially suitable for use in nuclear medicine. There is a growing interest in the use of 64 Cu, 62 Cu, 61 Cu, and 60 Cu for diagnostic purposes and 67 Cu and 64 Cu for targeted radiotherapy.
| Copper is often used for electrical wiring applications and for household plumbing applications. |
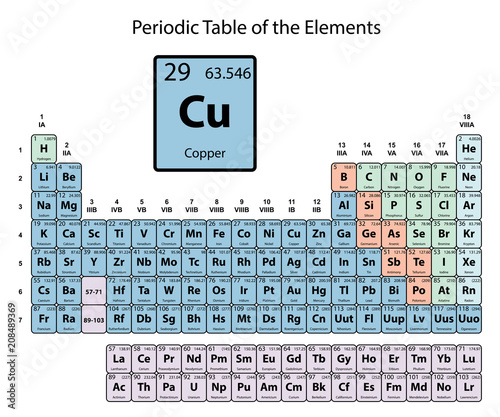
Copper
| Atomic Number: | 29 | Atomic Radius: | 140 pm (Van der Waals) |
| Atomic Symbol: | Cu | Melting Point: | 1084.6 °C |
| Atomic Weight: | 63.55 | Boiling Point: | 2562 °C |
| Electron Configuration: | [Ar]4s13d10 | Oxidation States: | −2, +1, +2, +3, +4 (a mildly basic oxide) |
History
From the Latin word cuprum, from the island of Cyprus. It is believed that copper has been mined for 5,000 years.
Properties

Copper is reddish and takes on a bright metallic luster. It is malleable, ductile, and a good conductor of heat and electricity (second only to silver in electrical conductivity).
Sources
Copper occasionally occurs natively, and is found in many minerals such as cuprite, malachite, azurite, chalcopyrite, and bornite.
Large copper ore deposits are found in the U.S., Chile, Zambia, Zaire, Peru, and Canada. The most important copper ores are the sulfides, the oxides, and carbonates. From these, copper is obtained by smelting, leaching, and by electrolysis.

Uses

The electrical industry is one of the greatest users of copper. Iron's alloys -- brass and bronze -- are very important: all American coins are copper alloys and gun metals also contain copper.
Copper has wide use as an agricultural poison and as an algaecide in water purification. Copper compounds, such as Fehling's solution, are widely used in analytical chemistry tests for sugar.
Availability
High-purity copper (99.999+ percent) is available commercially.
Click to see full answer
 Likewise, people ask, how many protons neutrons and electrons does copper have?
Likewise, people ask, how many protons neutrons and electrons does copper have?Copper has an atomic number of 29, so it contains 29 protons and 29 electrons. The atomic weight (sometimes called atomic mass) of an atom is approximated by the sum of the number of protons and the number of neutrons in the nucleus of the atom.
Isotopes Of Copper - Wikipedia
Furthermore, how many neutrons does the CU 63 isotope contain? It contains 29 protons and has a mass number of 63, as suggested in the name. In order to calculate the number of neutrons, you must take its mass number (63) away from its atomic number (29), which leaves you with 46. Therefore, Cu-63 contains 29 protons, and 46 neutrons.
Moreover, how many protons neutrons and electrons does copper 65 have?
How many protons, neutrons and electrons are there in a neutral atom of the isotope of copper named copper-65? Answer: protons: 29. neutrons: 36.
Atomic Number Of Copper
How do you find the number of protons neutrons and electrons?
Why Is Copper Denoted By The Symbol ‘Cu’?The Symbol ‘Cu’ Denotes The Latin Name Of Copper, Which Is Cuprum. Copper Can Be Classified As A Native Metal Which Can Occur In Nature In Its Pure...
Explanation: You can simply subtract the atomic number from the mass number in order to find the number of neutrons. If the atom is neutral, the number of electrons will be equal to the number of protons.